Plastics & Paper in Contact with Foodstuffs (P&P) is a leading meeting hub for the food contact industry. In recent editions, over 150 food contact representatives have joined the conference to gain the latest industry insights and network face-to-face with global experts.
The next conference will take place in 2026, with details to be announced soon. Register your interest to stay informed about the event.
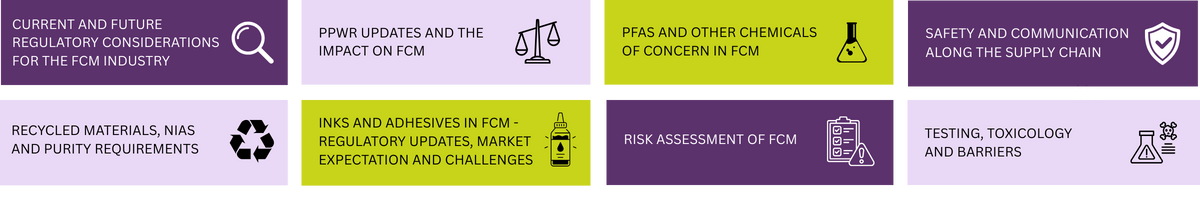
-------------------------------------------------------------------------------------------------------------------------------------
"The Plastics and Paper conference was a valuable experience, showcasing diverse perspectives from across the food and packaging industries, and service providers. The breadth of speakers fostered insightful discussions on the complex and rapidly evolving regulatory landscape."
Packaging Regulatory Compliance Specialist, GENERAL MILLS
-------------------------------------------------------------------------------------------------------------------------------------
"As always a good mixture of catching up the new and draft regulations, insight from associates from industry and some practical examples."
Senior Project Manager FCM, Ducares B.V. trading as Triskelion